| Method "Ethanol as Internal Standard" |

|

|
The theory of method
World market of alcoholic drinks is extensive and plays a great role in each state budgetary recharge. According to the statistics more than 500 million litres of spirits are produced worldwide every day. This fact leads us to understanding of high necessity of quality control of this production. Quantity of volatile compounds is the topmost quality parameter of these products. Further we will cite few legislative documents concerning methods of volatiles quantification with GC.
According to the legislations volatile compounds concentrations must be expressed in mg/L (or g/L, or g/hL) of absolute alcohol (AA).
Ethanol as Internal Standard
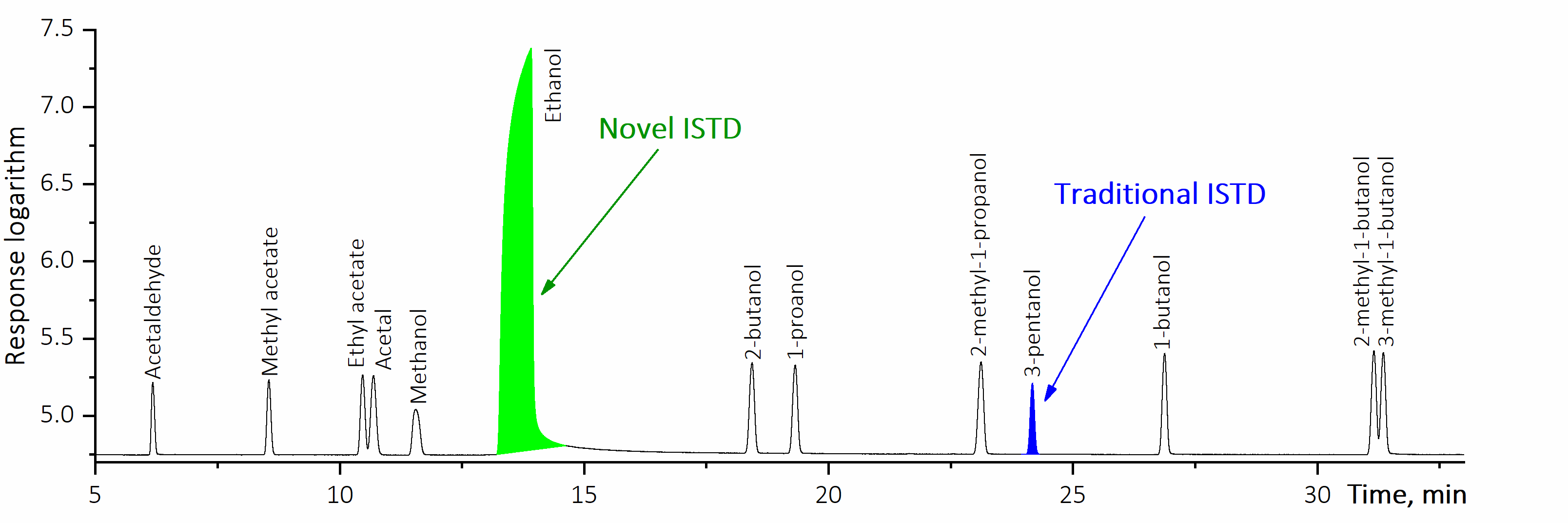
Oppositely to the generally adopted practices, the suggested “Ethanol as Internal Standard” method applies the major volatile compound as IS.
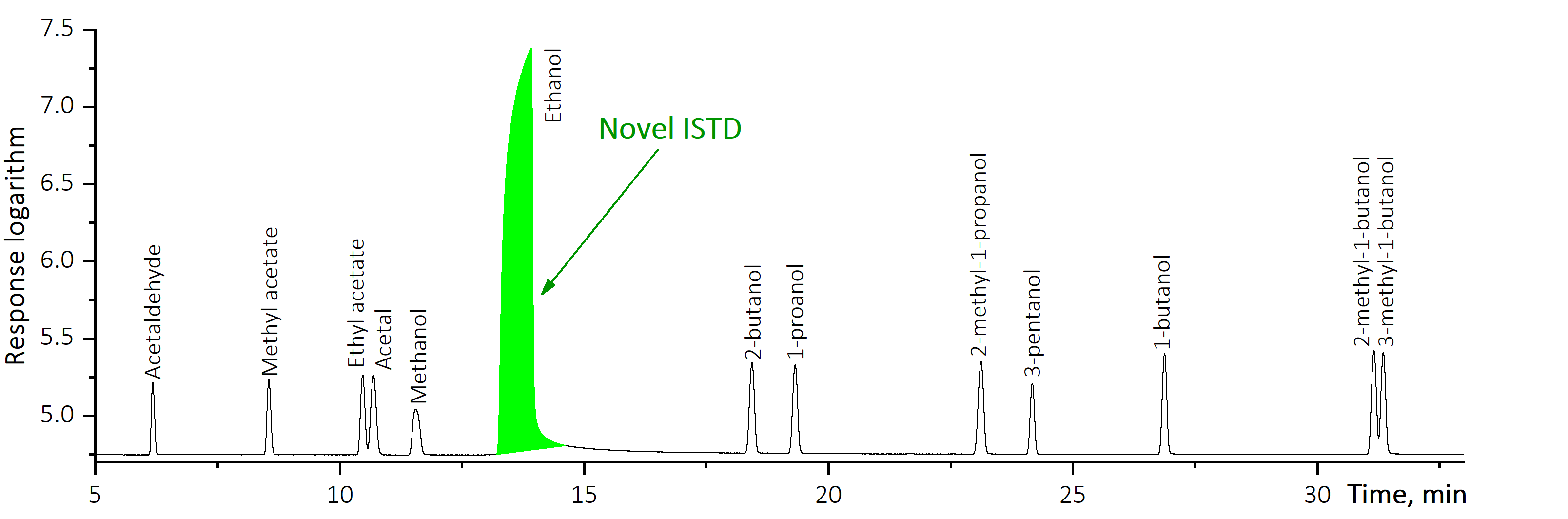
As ethanol always presents in alcohol-containing products, there is no necessity in its artificial addition into analysed sample. Also its concentration in mg/L AA units is known with a 100 % guarantee in each and every ethanol containing product and is equal to ethanol density. The process of sample analysis is similar to that in the traditional IS method as relative response factors (RRF) are to be determined:
|
|
(1) |
where ![]() and
and ![]() are the values of detector response, for example, peak areas, for the i-th individual compound and ethanol in standard solution, respectively;
are the values of detector response, for example, peak areas, for the i-th individual compound and ethanol in standard solution, respectively; ![]() is the concentration of the i-th analysed compound in mg/L AA units;
is the concentration of the i-th analysed compound in mg/L AA units;
![]() = 789300 mg/L is ethanol density.
= 789300 mg/L is ethanol density.
Method fully eliminates the necessity of sample density measurement and ABV determination as concentrations of volatiles in mg/L AA units are determined directly from GC data:
|
|
(2) |
Algorithm of application
The suggested method is very simple and can be easily used in each laboratory equipped with instrumentation necessary for traditional method application. The concrete algorithm of “Ethanol as IS” method usage is the following:
| Compound | Calibration | ||||
| Concentration "C", mg/L AA | Response 1, a.u. | Response 2, a.u. | Response3, a.u. | RRF | |
| Acetaldehyde | 428.250 | 26.548 | 26.676 | 26.955 | 1.224 |
| Methyl acetate | 682.510 | 34.498 | 33.961 | 34.613 | 1.517 |
| Ethyl acetate | 616.510 | 43.492 | 42.927 | 43.733 | 1.085 |
| Acetal | 552.980 | 52.283 | 51.473 | 52.633 | 0.810 |
| Methanol | 481.410 | 31.720 | 31.521 | 31.966 | 1.159 |
| Ethanol | 789300 | 60268 | 59835 | 60761 | 1.000 |
| 2-butanol | 497.890 | 60.212 | 59.785 | 60.769 | 0.631 |
| 1-propanol | 484.060 | 57.201 | 56.777 | 57.634 | 0.646 |
| 2-methyl-1-propanol | 474.360 | 66.381 | 65.845 | 66.873 | 0.546 |
| 3-propanol | 536.020 | 71.437 | 70.809 | 71.924 | 0.573 |
| 1-butanol | 496.770 | 64.686 | 64.272 | 65.238 | 0.586 |
| 2-methyl-1-butanol | 514.010 | 72.918 | 72.392 | 73.344 | 0.539 |
| 3-methyl-1-butanol | 492.710 | 69.622 | 69.173 | 70.083 | 0.541 |